Learning Outcomes
By the end of this lesson, students should be able to:
i. Analyze IR spectra and identify the characteristic absorption bands of common functional groups, such as C-H stretching, C-H bending, C=O stretching, O-H stretching, and C≡C stretching.
ii. Relate the observed IR absorption bands to the presence of specific functional groups in the molecule.
iii. Apply their knowledge of IR spectroscopy to determine the structures of simple organic compounds, such as phenol, toluene, acetone, and ethanol, from their IR spectra.
iv. Appreciate the importance of IR spectroscopy as a valuable tool for structure elucidation in organic chemistry.
Introduction
Infrared (IR) spectroscopy, a powerful analytical technique, provides a wealth of information about the structure and functional groups of molecules through the analysis of their absorption of infrared radiation. This lesson delves into the realm of structure determination from IR spectra, guiding students through the process of identifying functional groups and deducing the structures of simple organic compounds.
i. Functional Groups: The Building Blocks of IR Spectra
Functional groups, specific arrangements of atoms within a molecule, exhibit characteristic IR absorption bands, providing a molecular fingerprint.
C-H Stretching: Strong absorption near 3000 cm-1, indicating the presence of alkyl groups.
C-H Bending: Weaker absorption near 1400 cm-1, also indicating alkyl groups.
C=O Stretching: Strong absorption in the range of 1700-1750 cm-1, characteristic of carbonyl compounds (aldehydes, ketones, carboxylic acids).
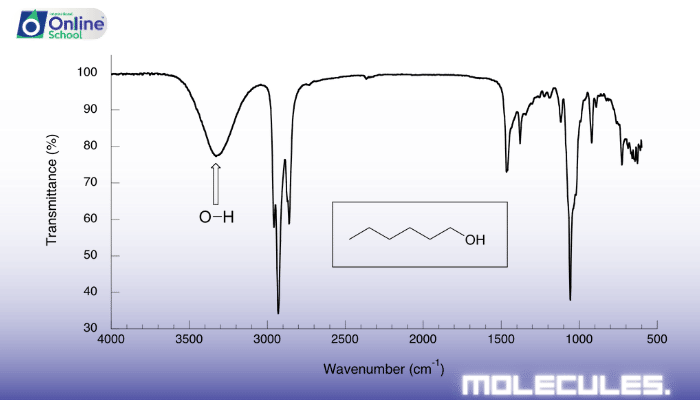
O-H Stretching: Broad absorption near 3300 cm-1, indicating alcohols and carboxylic acids.
C≡C Stretching: Characteristic absorption near 2200 cm-1, indicating alkynes.
ii. Phenol: Unraveling the Aromatic Ring
Phenol, a benzene ring with an attached hydroxyl group, exhibits the following IR absorption bands:
- Strong C-H stretching absorption near 3000 cm-1
- Medium C=C stretching absorption near 1600 cm-1
- Strong O-H stretching absorption near 3300 cm-1
iii. Toluene: The Methylated Benzene
Toluene, a methyl-substituted benzene, exhibits the following IR absorption bands:
- Strong C-H stretching absorption near 3000 cm-1
- Medium C=C stretching absorption near 1600 cm-1
- Weak C-H bending absorption near 1450 cm-1
iv. Acetone: The Ketone Symphony
Acetone, a ketone with two methyl groups, exhibits the following IR absorption bands:
- Strong C-H stretching absorption near 3000 cm-1
- Weak C=O stretching absorption near 1700 cm-1
- Weak C-H bending absorption near 1430 cm-1
v. Ethanol: Unveiling the Alcohol
Ethanol, a simple alcohol, exhibits the following IR absorption bands:
- Strong C-H stretching absorption near 3000 cm-1
- Broad O-H stretching absorption near 3300 cm-1
- Weak C-O stretching absorption near 1050 cm-1
IR spectroscopy, through its analysis of IR absorption bands and the identification of characteristic functional group signatures, provides an invaluable tool for structure elucidation in organic chemistry. By carefully interpreting IR spectra, chemists can unravel the molecular structures of organic compounds, gaining insights into their properties and reactivity.